The reason why graphite and diamond have different properties is because of the different ways carbon atoms are arranged in their crystal structures. In the case of diamond, the carbon atoms are arranged in a tetrahedral structure, while in graphite, they are arranged in 2D sheets. These different arrangements lead to differences in the chemical and physical properties of the two allotropes.
Diamond and graphite are both forms of carbon, but their properties differ because of the way their crystal structures are arranged. Although they are made of the same element, carbon, they have distinct differences in their properties. Despite being allotropes of the same element, they do not share many similarities.
At first glance, the combination of diamond and graphite may seem odd. Diamond is often associated with precious gemstones like gold or sapphire, while graphite is commonly found in pencils. So why are they grouped together?
If you paid attention in your high school Chemistry lessons, you would know that there is a strong structural connection between diamond and graphite.
What is that connection, and why are they so different from each other?
What Are Allotropes?
Allotropy, also known as allotropism, refers to the ability of an element to exist in multiple forms with different arrangements of its atoms while remaining in the same physical state. These different forms are called allotropes of the element.
Imagine you have 36 balls that can be arranged in various patterns to create visually different shapes.
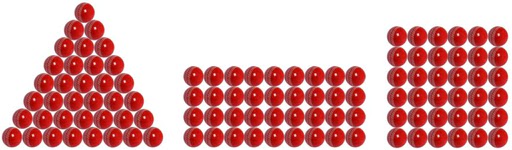
Each ball represents an atom, and the different shapes they form due to their arrangements are the allotropes.
Allotropes of the same element have different bonding arrangements, resulting in different chemical and physical properties. They can also differ in the number of atoms in a molecule.
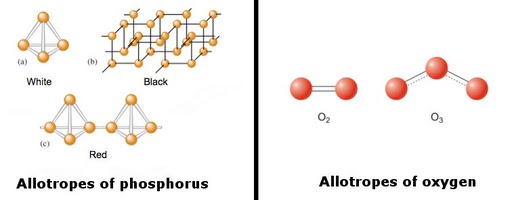
The image below shows different allotropes of phosphorus and oxygen.
Allotropes Of Carbon
Carbon is a rockstar in the world of allotropes. It can form numerous allotropes due to its chemical structure. With an atomic number of 6, carbon has 4 electrons in its valence shell.

Currently, there are at least 8 known allotropes of carbon, and research is ongoing to discover more.
The carbon atoms in diamond are arranged in a tetrahedral manner, meaning each carbon atom is connected to four other carbon atoms through strong covalent bonds. This arrangement gives diamond its characteristic strength, durability, and rigidity. Diamond is one of the hardest naturally-occurring materials on Earth, requiring a high amount of force to scratch or break.
In contrast, graphite has a different geometric arrangement. Its carbon atoms form 2D sheets, with each carbon atom bonded to three others to create hexagonal rings. While the bonding within each layer is covalent and strong like in diamond, the bonding between layers is weak, relying on Van der Waals forces.
As a result, the layers in graphite can easily slide over each other and detach. This weak bonding allows graphite to flake off on paper, making it suitable for writing with pencils. Graphite is also soft, slippery, and has a lower density compared to diamond.
What amazes me the most is how slight changes in the chemical structure of identical substances can result in such massive differences in appearance, toughness, and chemical properties.
