To put it simply, when wood comes into contact with fire, it undergoes thermal degradation, or pyrolysis. This pyrolysis of wood results in the release of specific volatile gases and the creation of char, which then undergoes flaming and glowing reactions, respectively, in order to release heat energy.
If you are the type of adventurous individual who enjoys camping in the wilderness, then you may have also enjoyed sitting around a campfire, savoring the sweet deliciousness of s’mores. If this sounds like you, then you should be grateful for the wood that had to burn in order for you to enjoy the treat.
 (Photo Credit: pixabay)
(Photo Credit: pixabay)
We are all familiar with what occurs when wood encounters fire. It initially produces smoke and then bursts into flames with shades of orange and red. The pile of glowing embers eventually dies down, leaving behind a heap of cooled grey ashes.
All of this is what we observe on the surface level, but have you ever considered what happens at the molecular level of the wood during its fiery demise?
Wood Composition
The chemical makeup of wood varies depending on the part, type, and location of the tree it is derived from. However, in general, wood consists of two main chemical components: lignin (18-35%) and carbohydrates (65-75%). Carbohydrates, such as cellulose (40-50%) and hemicellulose (25-35%), make up the majority of the wood’s composition.
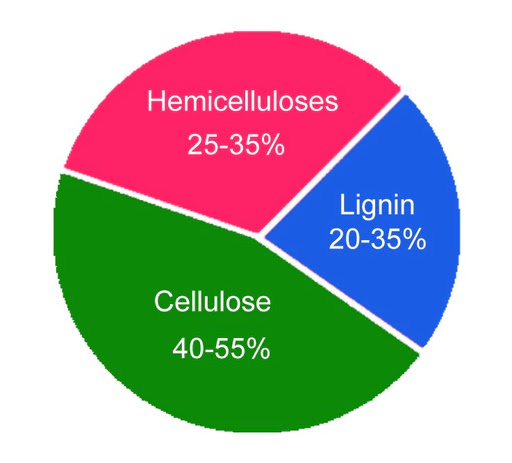 Chemical Composition of wood.
Chemical Composition of wood.
In addition to these complex molecules, wood may also contain inorganic substances (ash) and organic extractives (phenolic compounds, fats, waxes, terpenes, and terpenoids).
In summary, wood has a basic composition of approximately 50% carbon, 6% hydrogen, 44% oxygen, and trace amounts of metal ions. Almost all wood species have a similar elemental composition.
Wood Burning Process
When wood is set on fire, it undergoes a combustion reaction rather than simply burning. This may seem confusing, so what is the difference between combustion and burning?

In order for combustion to occur, a fuel like wood must be oxidized to release energy in the form of heat, without producing flames. On the other hand, burning (which is a type of combustion) releases energy in the form of light (flame). Since most of the energy is converted into light, the amount of heat generated is relatively less compared to combustion.
Simply put, when wood comes into contact with fire, it undergoes thermal degradation or pyrolysis.
Wood Pyrolysis
Pyrolysis plays a significant role in the combustion of wood. It is the process of breaking down organic matter through heating in the absence of oxygen or under an inert atmosphere. Wood pyrolysis results in the release of volatile gases and the formation of char, which then undergo flaming and glowing reactions to release heat energy. The pyrolysis of wood can be divided into three main stages.
Stage 1
To initiate the combustion of wood, heat is applied to the wood in the presence of air. This causes a localized increase in temperature on the wood surface. When the temperature reaches 100°C, the water in the wood begins to boil and evaporate. Since wood with high moisture content is difficult to burn, dehydration is necessary for combustion to occur. Dehydration is typically completed at around 160°C.
 Combustion of wood releases gases such as carbon dioxide and water vapors (Photo Credit: Nasky/Shutterstock)
Combustion of wood releases gases such as carbon dioxide and water vapors (Photo Credit: Nasky/Shutterstock)
Between 200°C and 280°C, the hemicellulose in the wood starts to decompose, producing gases such as carbon dioxide, carbon monoxide, acetic acid, and formic acid.
The gases released during the first stage do not ignite until the moisture has completely evaporated and the temperature is sufficiently high.
Stage 2
The second stage of wood combustion is the heat-producing stage. It begins when the temperature of the wood exceeds 280°C. During this stage, a significant amount of energy is released along with unburnt combustible gases like methane and methanol, water vapor, and carbon dioxide. These gases, known as secondary gases, make up about 60% of the potential heat in the wood and are crucial for efficient wood combustion.
In this stage, cellulose starts to decompose and reaches its peak decomposition temperature at around 320°C. The decomposition of cellulose leads to the formation of char, tar, and volatile products.
Stage 3
When temperatures go beyond 320°C, the rate at which lignin breaks down becomes stronger. At this point in the combustion of wood, all the gaseous products evaporate. These gases mix with air and either cool down to form smoke or ignite and burn in flames. The only solid product left behind from the pyrolysis of wood is the carbon chains of cellulose and lignin molecules, which turn into charcoal. About 20-30% of the weight of the wood is converted into charcoal.
 A stack of glowing charcoal (Photo Credit: Buncha Lim/Shutterstock)
A stack of glowing charcoal (Photo Credit: Buncha Lim/Shutterstock)
The bed of charcoal continues to burn for a long time, but with low heat output. In the absence of oxygen, the glowing charcoal loses its energy and cools down. However, blowing air onto the glowing charcoal causes it to burn brighter and spread the fire. Therefore, supplying new oxygen to the charcoal bed and adding more wood can reignite the fire.
After all the combustible products are removed, a small amount of wood that did not burn, known as ash, is left behind. Wood ash contains potassium carbonate, which acts as a good soil fertilizer.
Conclusion
Until now, you might have thought that burning wood is a simple process where fire consumes every molecule it encounters. However, it is evident that things are not as simple as they might seem!

When wood comes into contact with fire, it triggers a series of complex chemical reactions. The combustion of wood releases carbon dioxide, water vapor, and various gaseous products, while also forming black solid residues like charcoal and ash.
