According to Niels Bohr’s Atomic Theory, an atom can be compared to a planetary model, with electrons located in discrete and energized orbits. When an excited electron jumps from a higher orbit to a lower orbit, the atom emits a photon. The energy difference between these orbits is equal to the energy of the photon.
Niels Bohr, a Danish physicist, is recognized as one of the key figures in the development of quantum mechanics, particularly the old quantum mechanics. As a token of appreciation for his significant contributions to science, the Carlsberg brewing company granted him a house located adjacent to one of their breweries. The house was connected to the brewery through a pipeline, allowing Bohr to have a lifetime supply of free beer on tap whenever he desired. What extraordinary achievement did Niels Bohr accomplish to receive this prestigious honor, along with a Nobel Prize?
 Niels Bohr. (Photo Credit: Public domain / Wikimedia Commons)
Niels Bohr. (Photo Credit: Public domain / Wikimedia Commons)
In essence, Niels Bohr shed light on the enigmatic workings of the atom. Although he collaborated with Ernest Rutherford, the pioneering figure in the discovery of the atomic nucleus, the model is solely attributed to Bohr. Initially referred to as the Rutherford-Bohr atomic model, it is now commonly known as Bohr’s atomic model.
In order to comprehend Bohr’s theory, we must first grasp the prior breakthroughs that motivated him to pursue his revolutionary ideas.
Rutherford’s Unsuccessful Model
It was Sir J.J. Thomson who initially revealed that the atom was not indivisible, contrary to the long-held belief. However, the subatomic particle he discovered had a negative charge. If atoms were simply conglomerates of negative charges, stability would be non-existent for objects such as chairs, tables, and even ourselves. Thomson promptly recognized that in order to account for matter’s stability, there must be a net positive charge to counterbalance the negativity.
Thomson proposed the very first model of an atom, suggesting that the negatively charged particles, known as electrons, were like seeds embedded in a positively charged watermelon. This model is commonly referred to as the plum or raisin pudding model. The analogy is quite self-evident.

This perspective prevailed until Ernest Rutherford demonstrated that when positive particles were directed at an atom, most of them passed straight through, while a few were deflected at significant angles. Rutherford concluded that the majority of the atom consisted of empty space, with a dense, point-like concentration of positive charge at its center. This central region was termed the nucleus. The volume of empty space between an atom’s electrons and its nucleus is so vast that if the atom were enlarged to the size of a baseball stadium, the nucleus would be the size of a baseball.
Rutherford proposed that the atomic system may be analogous to our Solar System, where electrons orbit the nucleus akin to planets revolving around the Sun. The significant difference, of course, is that the electrons are held by electrostatic force rather than gravity. However, Maxwell and Hertz would have vehemently disagreed.
Rutherford’s model of the atom was based on the understanding that the motion of charged particles, such as electrons, requires energy. This meant that an electron revolving around the nucleus would eventually lose all its energy and collapse. However, a new atomic model was needed to explain why matter is stable.
Another puzzle for physicists at the time was the emission spectrum of different atoms, which was discovered through Planck’s black body radiation. When an object is heated, it emits a spectrum of electromagnetic energy. For example, as a bar of iron is heated, its color changes gradually from red to orange to bright white and then towards violet. This change in color is due to the range of visible light that is emitted by the iron bar.
Every object in the universe, including humans, emits a spectrum of energy. However, our eyes can only detect a small portion of this spectrum, which falls within the range of visible light. Planck referred to this phenomenon as black body radiation. The intensity of heat and the wavelength of the light emitted are related, with different objects having peaks at different wavelengths.
As the temperature of a body decreases, the wavelength of the light it emits increases. For example, the radiation from the Big Bang started as gamma rays but has cooled down over billions of years to become microwaves. If these waves are plotted on a black background, a continuous spectrum of colors can be observed.
Planck’s discovery also revealed that the energy radiated by objects travels in discrete packets called photons. The energy of a single quantum is inversely proportional to its wavelength or directly proportional to its frequency. This relationship is described by Planck’s constant, denoted as “h”. The energy (E) for a frequency (v) can be expressed as E = hv.
When a volume of gas consisting of a single element is heated and the colors are plotted on a black background, an interesting phenomenon occurs. The spectrum is no longer a continuous blend of colors, but rather a series of distinct, single-colored lines with black intervals in between. One example of this is the well-known spectrum of hydrogen.
In fact, every element in the Universe has its own unique and discontinuous spectrum. While hydrogen’s spectrum falls within the visible range, other elements have spectra that lie in the ultraviolet or infrared range. This uniqueness of each element’s spectrum allows scientists to study the composition of stars and has even led to the discovery of new elements.
Upon examining the spectrum of hydrogen, it becomes clear that only specific colors appear because only certain frequencies associated with these colors are emitted. This raises the question of why atoms exhibit this peculiar behavior and what atomic structure restricts them to express themselves in such a limited manner. Niels Bohr, in 1913, provided an explanation for this.
Bohr built upon Rutherford’s Solar System model of the atom but made a small modification. He suggested that electrons revolve around the nucleus in fixed orbits, without losing any energy and thus preventing a collapse into the nucleus. These fixed orbits, which Bohr called “stationary orbits,” were not randomly positioned but instead located at specific distances from the central nucleus, each associated with a fixed energy. Inspired by Planck’s theory, Bohr used the quantum number “n” to represent these orbits.
Despite its seemingly absurd nature, Bohr’s theory accurately predicted the spectrum of hydrogen. According to his theory, when a gas is heated, its energized electrons jump from an orbit of lower energy to one of higher energy (for hydrogen, from n = 1 to n = 2). To regain stability, the electrons must then jump back down to lower energy orbits. During this transition, the electron releases energy in the form of light.
The discrete nature of the orbits provides an explanation for the discrete nature of photons. Bohr discovered that the energy of an emitted photon is equal to the difference in energies between the two levels the electron transitions between. For example, infrared light is emitted during a shorter leap, while ultraviolet light is emitted during a larger leap. This relationship can be expressed as “E2 – E1 = hv.” Conversely, when an electron absorbs a photon, it jumps to a higher orbit.
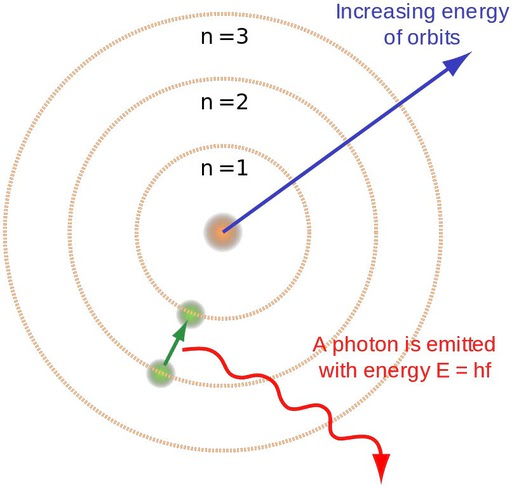 When an electron moves from a higher orbit to a lower orbit, it releases a photon. (Photo Credit: Brighterorange / Wikimedia Commons)
When an electron moves from a higher orbit to a lower orbit, it releases a photon. (Photo Credit: Brighterorange / Wikimedia Commons)
An atom’s spectrum is limited to specific colors because its organized structure allows its electrons to only undergo certain energy transitions and therefore emit certain frequencies of light. However, even though a hydrogen atom only has one electron, its spectrum consists of multiple colors. This is because the gas is composed of countless atoms with electrons occupying different orbits that are either higher or lower than those nearby.
This was Bohr’s planetary model, where electrons were located in discrete orbits with specific energy levels. When an excited electron in the atom jumped from a higher orbit to a lower orbit, it emitted a photon. The energy difference between these orbits was equal to the energy of the photon.
Limitations
Unfortunately, Bohr’s model could only explain the behavior of a system with two charged particles orbiting each other. This made it applicable only to the hydrogen atom. It also applied to ionized helium (where one electron was removed, leaving only one) or double-ionized lithium (with three electrons). His theory couldn’t account for the behavior of any other atom besides hydrogen.
 Bohr’s model could only explain the behavior of a system with two charged particles orbiting each other, particularly the hydrogen atom.
Bohr’s model could only explain the behavior of a system with two charged particles orbiting each other, particularly the hydrogen atom.
Additionally, his theory suggested that electrons aligned themselves in stationary orbits like beads on a string, assuming a non-interacting system of electrons. This completely ignored the repulsive electrostatic force between multiple electrons clustered together, which would push them apart. Eventually, it was discovered that electrons not only revolve around the nucleus but also rotate or spin on their own axis. Bohr’s model couldn’t explain why this spin didn’t result in a loss of energy.
It is believed that one reason Bohr’s theory was widely accepted is that it made successful theoretical predictions about various spectra that hadn’t been observed yet. Despite its limitations, it is highly praised for revolutionizing modern physics and paving the way for modern quantum mechanics. Eventually, modern quantum mechanics provided a complete explanation for the true nature of energy shells, how electrons occupy them, and the issue of spin.
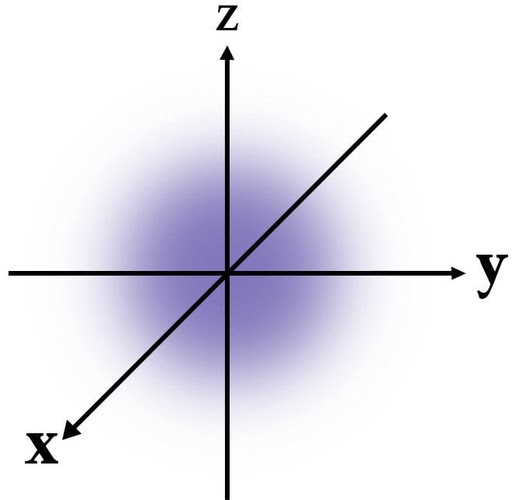 According to quantum mechanics, the exact location of an electron within an energy shell cannot be determined. Instead, it is more probable to find the electron in a certain shell. The image shows the first energy shell, with the density of blue increasing towards the center, indicating that the electron is most likely to be found near the nucleus.
According to quantum mechanics, the exact location of an electron within an energy shell cannot be determined. Instead, it is more probable to find the electron in a certain shell. The image shows the first energy shell, with the density of blue increasing towards the center, indicating that the electron is most likely to be found near the nucleus.
However, despite its simplicity, Bohr’s ideas still prevail and dominate high school physics. Textbooks often depict concentric circles filled with electrons surrounding a nucleus, resembling the beads-on-a-string model. For his contributions, Bohr certainly deserved that free beer and, of course, a Nobel Prize.
