Experiments conducted by Henry Moseley revealed that each element has a unique atomic structure that interacts with X-rays in a distinct way.
Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon…
These words will bring back memories of our chemistry class, where we were required to memorize the elements of the periodic table, at least up to element 20. Although this practice seemed to defeat the purpose of having a periodic table, it turned out to be quite useful. We were also taught that elements in the periodic table are arranged based on their atomic numbers, which represent the number of protons in an atom, and also the number of electrons.
But how did scientists figure this out? Since atoms are extremely small, it is impossible to count them visually. So, how did the scientists who developed the periodic table determine what an atomic number was?
This discovery was made thanks to a fortuitous winter in Germany and a young, brilliant scientist from the University of Manchester.
Röntgen And His Discovery
Some may wonder why scientists didn’t simply use a microscope to observe atoms and count the number of protons. Well, even the most powerful optical microscope cannot visualize an atom. We can only see objects that disrupt the path of light waves and reflect them into our eyes.
An atom is 10,000 times smaller than the wavelength of visible light, so it has no effect on the waves. Imagine a grain of sand standing against a giant ocean wave (not to scale).
The exploration of subatomic particles received a significant boost after Wilhelm Röntgen discovered X-rays. It was during the winter of 1895 when Röntgen, like many other scientists of his time, was studying the rays emitted by the Crooke’s tube.
The Crooke’s tube, also known as a cathode ray tube, is a sealed glass vacuum chamber containing two electrodes. When voltage is applied across the electrodes, the tube emits a faint glow.
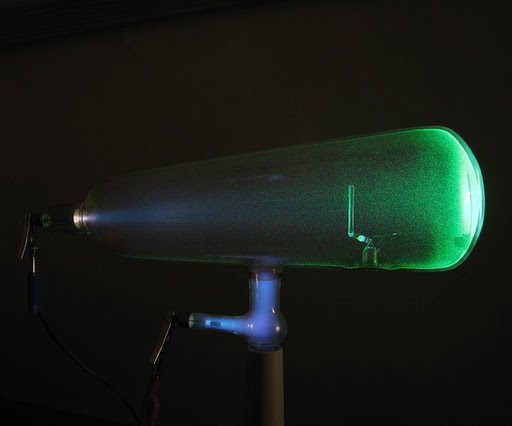 A Crookes tube glowing in a dark room (Photo Credit : D-Kuru/Wikimedia commons)
A Crookes tube glowing in a dark room (Photo Credit : D-Kuru/Wikimedia commons)
Röntgen had his “Eureka” moment when he noticed that the rays emitted by the tube were causing bright spots on a platinobarium screen located nearly 9 feet away from the setup. To test the penetrating abilities of these invisible rays, he covered the tube with thick black cardboard, yet the glow was still visible on the screen. He named these unknown rays X-rays.
On the 22nd of December, just three days before Christmas, Röntgen placed his wife Anna’s left hand on a piece of photographic paper and captured the world’s first X-ray image of bones. The rays passed through her skin but were stopped by her bones and her wedding ring. Astonished by the dark silhouette of her hand on the photographic plate, she exclaimed, “I have seen my death,” as this was the first time in history that a living person had seen their own skeleton.
The very first X-ray image of a human hand was taken. X-rays quickly became a significant scientific discovery, revolutionizing science and captivating the general public with exhibitions showcasing their capabilities. X-rays were able to solve the problem of atomic numbers because their wavelengths were smaller than the size of an atom, allowing them to interact with atoms. X-rays were discovered to be electromagnetic waves with higher energy than visible light, capable of penetrating objects that light could not. This new investigative tool brought together chemists, biologists, and physicists, revealing the skeletal structure of living organisms and the arrangement of atoms in crystals through X-Ray diffraction crystallography. Henry Moseley, who was initially appointed by Ernest Rutherford to study radioactive elements, became fascinated with X-ray spectroscopy and began working independently. Inspired by a study proposing that elements should be arranged based on the charge in their atomic nucleus rather than atomic weight, Moseley used X-ray spectroscopy to experimentally test this hypothesis. By analyzing the unique spectra produced by different elements when exposed to X-rays, Moseley discovered that the square root of the X-ray frequency emitted by an element was proportional to Z-1, where Z represents the charge of the atomic nucleus. This led to the concept of atomic numbers and the rearrangement of the periodic table. Unfortunately, Moseley’s promising scientific career was cut short when he lost his life during World War I. Ernest Rutherford believed that Moseley would have undoubtedly received a Nobel Prize if not for his untimely death.
Summary
The discovery of quantum mechanics has demonstrated that the distinct X-ray spectra were a result of quantized electronic transitions rather than nuclear charge. Nevertheless, Moseley’s experiments indirectly provided us with a glimpse into the inner workings of an atom and its implications on the external world.
With the assistance of Scanning Tunneling Microscopes, we are now able to visualize the structure of an atom. However, we are still a long way from being able to physically dissect an atom and determine the exact number of subatomic particles it contains. Despite being more than a century since Moseley introduced atomic numbers, they continue to shape our understanding and manipulation of elements in the captivating field of chemistry.
