Anomers and epimers are both types of diastereomers. However, an epimer is a stereoisomer that has a different configuration at any single stereogenic center, while an anomer is actually an epimer that has a different configuration at the acetal/hemiacetal carbon.
Before discussing the differences between epimers and anomers in more detail, it is important to first understand what stereoisomers are.
Definition of Stereoisomer
To understand what a stereoisomer is, it is crucial to have some knowledge about isomerism in general. An isomer is a molecule that has the same molecular formula as another molecule, but possesses different chemical properties. In other words, isomers have the same number of atoms of each element, but the arrangement of these atoms is different.
 The different types of isomers, such as position isomers 2-fluoropropane and 1-fluoropropane on the left (Photo Credit : Vladsinger / Wikimedia Commons)
The different types of isomers, such as position isomers 2-fluoropropane and 1-fluoropropane on the left (Photo Credit : Vladsinger / Wikimedia Commons)
There are two types of isomerism: structural isomerism (where functional groups are attached in different ways) and stereoisomerism.
Definition of Stereoisomerism
Stereoisomers are isomeric molecules that have the same molecular formula, but different 3-D orientations of their constituent atoms in space. Stereoisomers are further classified into two types: enantiomers and diastereomers.
Enantiomers are stereoisomers that are non-superimposable mirror images of each other. Also known as optical isomers, they have similar physical properties.
 (S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are nonsuperimposable mirror images of each other (Photo Credit : NEUROtiker / Wikimedia Commons)
(S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are nonsuperimposable mirror images of each other (Photo Credit : NEUROtiker / Wikimedia Commons)
Diastereoisomers are stereoisomers that have different configurations at one or more (but not all) stereoisomers without being mirror images of each other. The term ‘epimer’ is used to refer to diastereomers that differ in configuration at only one chiral center.
An anomer is a type of epimer that differs in configuration, especially at the acetal/hemiacetal carbon.
Comparison: Epimer vs Anomer
While an epimer is one of a pair of stereoisomers that differ in configuration at only one chiral (stereogenic) center, an anomer is actually an epimer (also a cyclic saccharide) that differs in configuration, especially at the acetal or hemiacetal carbon (refer to the image below to differentiate between acetal and hemiacetal carbons).
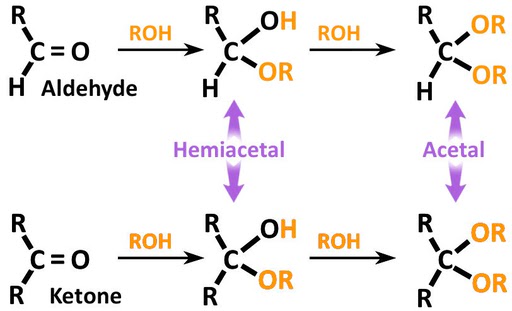
An epimer is one of a pair of stereoisomers that differ in configuration only at the chiral center. All other stereocenters (if present) are the same in both molecules. If the pair of molecules has only 1 stereocenter, then the epimers are enantiomers. However, if the molecules have 2 or more stereocenters, the epimers are referred to as diastereomers. (Source)
Refer to the following figure with two stereoisomers of chlorobutane.
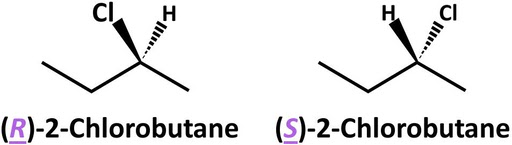
(R)-2-Chlorobutane and (S)-2-chlorobutane differ in absolute configuration at the C2 stereocenter. Note that 2-chlorobutane has only one stereocenter, which is why these epimers are enantiomers.
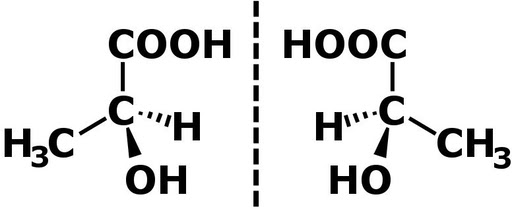
On the other hand, in the stereoisomeric structures of tartaric acid, you can observe that the two epimers (i.e., (2R,3R)-Tartaric acid and (2R,3S)-tartaric acid) differ in absolute configuration at the C3 stereocenter.

Note that tartaric acid has two stereocenters, which is why these epimers are diastereomers.
Anomers are a type of stereoisomer that refers to saccharides or glycosides that are epimers. Epimers are different from each other in the arrangement of either the C-2 configuration (for ketoses) or the C-1 configuration (for aldoses).
In many cases, carbohydrates can exist in both cyclic and acyclic forms. When cyclization occurs, the carbon in the carbonyl group becomes a new stereocenter. This cyclization results in the formation of two diastereomers, which differ in the position of a specific functional group. The new stereocenter is called the “anomeric carbon”.
The image below helps to illustrate this process.
[image_2295.jpg]
The two anomers are referred to as “alpha” and “beta” based on their relationship to the anomeric reference atom. If the hydroxyl group on C-1 and the -CH2OH group on C5 are on opposite sides of the six-membered ring, C1 is the α anomer. If they are on the same side, C1 is the β anomer.
Example: α-D-Glucopyranose and β-D-glucopyranose.
[image_2301.jpg]
It is important to note that the two stereoisomers in the image above differ in the configuration of C-1.
In conclusion, both epimers and anomers are types of stereoisomers, with anomers being a special case of epimers. The main difference between them is that epimers differ in configuration at only one chiral center, while anomers differ in configuration, particularly at the acetal or hemiacetal carbon.
